HKU Achieves First Clinical Use of Next-Generation Infrared Fluorescence Imaging to Help Surgeons See Blood Perfusion During Esophageal Surgery
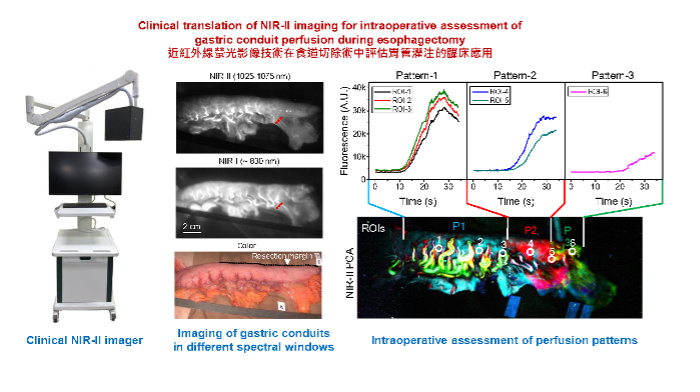
Figure 1. Left: Photograph of NIR-II fluorescence imager (AlbertSurgicalTM). Middle: Imaging of gastric conduits in different spectral windows. Right: Intraoperative principal component analysis (PCA) of the NIR-II video revealed distinct perfusion patterns in the gastric conduit. Image modified from Wang et al, PNAS (2025).
Esophagectomy is a complex surgical procedure involving the resection of a diseased or cancerous portion of the esophagus. One of its most dreaded complications is anastomotic leakage, a gastrointestinal defect that occurs at the suture line between the esophagus and the gastric conduit. This complication affects 10-30% of patients and leads to a high postoperative morbidity rate and even death.
A cross-disciplinary research team led by Professor Hongjie DAI (Sapientia Eminence Professor, Department of Chemistry, Department of Mechanical Engineering and School of Biomedical Sciences, Director of The Materials Institute of Life Sciences and Energy (MILES), The University of Hong Kong (HKU)) and Professor Simon Ying Kit LAW (Chairperson and Cheung Kung-Hai Professor in Gastrointestinal Surgery, Department of Surgery, the School of Clinical Medicine, HKUMed) has, for the first time, demonstrated how NIR-II (1000–3000 nm) fluorescence video imaging can be used during esophagectomy to guide surgical decision-making intraoperatively. By combining high-clarity NIR-II video imaging with rapid computational video analysis, the technology enables operator-independent objective delineation of boundaries between well-perfused and poorly perfused regions, providing a guide to resection and reconstruction with a reduced anastomotic leakage rate. In Hong Kong, about 30 patients have already benefited from esophagectomy performed with the aid of the new fluorescence imaging and video analysis technique, and the outcomes so far have been highly promising. The team’s findings have been published in Proceedings of the National Academy of Sciences (PNAS).
Accurate, operator-independent assessment of tissue perfusion and selection of an optimal anastomotic site during esophagectomy are essential for surgeons to ensure the viability of the gastric conduit and to minimise the risk of anastomotic leakage. Currently, near-infrared (NIR) fluorescence imaging using conventional dyes, such as indocyanine green (ICG), with a commercially available near-infrared I (NIR-I, 700-900 nm) instrument is used for diagnostics and intraoperative navigation.
Despite its widespread use, NIR-I angiography has several limitations, such as superficial penetration depth, high background signal and lower resolution caused by light scattering and tissue autofluorescence. Further, assessing perfusion based on the suboptimal images relies heavily on the surgeon’s experience and subjective image interpretation, making it difficult to precisely determine the demarcation line of blood perfusion in the gastric conduit, a process critical to the outcome of the surgery.
Intraoperative NIR-II fluorescence-guided esophagectomy in the operating room
This study, led by Professors Dai and Law, represents the first clinical use of NIR-II (1000 -3000 nm) fluorescence imaging to visualise blood perfusion in the gastric conduits, affording markedly superior contrast and resolution compared to traditional NIR-I imaging. NIR-II imaging was pioneered by the Dai’s team in 2009 with mice, taking advantage of the reduced light scattering and tissue autofluorescence at light wavelengths above 1,000 nm. This breakthrough marks the first successful translation of NIR-II imaging into clinical practice for upper gastrointestinal surgery in human patients.
Highlights of the study include, (1) the building of an imaging system that captures photographic images and NIR-II fluorescence images at the same time, (2) much clearer blood flow images in NIR-II than traditional NIR-I, allowing more precise assessment of blood perfusion, and (3) the combined use of a rapid intraoperative computational analysis of high-resolution blood perfusion NIR-II videos that identifies well-perfused and poorly perfused regions of the gastric conduits within one minute of dye administration.
Professor Hongjie Dai commented, “This is a great example of cross-disciplinary collaboration for translation of our imaging technique from mouse to man.” Professor Simon LAW remarked, “This innovation offers objective guidance for surgical decisions in esophagectomy. It enables surgeons to accurately assess blood supply, remove the poorly perfused portion in order to perform the anastomosis at a well perfused area, thereby reducing the risk of anastomotic leakage. This not only enhances surgical safety but also represents a significant breakthrough in esophagectomy.”
This study is part of Hong Kong’s Research, Academic and Industry Sectors One-plus Scheme (RAISe+), the JC STEM Lab of Nanoscience and Nanomedicine and Materials Institute of Life Sciences and Energy (MILES) in Shenzhen. This RAISe+ project is revolutionising surgery with advanced dyes and imaging tools that work in the NIR-II window. This technology gives surgeons “infrared vision” to see tumours, blood vessels, and organs clearly and deeply during operations. The team’s solution helps overcome current limitations of shallow imaging depth and low image quality, enabling precise tumour removal, ureter detection, and sentinel lymph node identification, making surgeries safer and more effective for patients.
The research team comprises the following HKU academics:
Professor Hongjie Dai, Sapientia Eminence Professor and Chair Professor, Department of Chemistry, Faculty of Science; Department of Mechanical Engineering, Faculty of Engineering; School of Biomedical Sciences, LKS Faculty of Medicine; and Materials Institute of Life Sciences and Energy (MILES), HKU.
Professor Simon Ying Kit Law, Chairperson, Chair Professor and Cheung Kung-Hai Professor in Gastrointestinal Surgery, Department of Surgery, School of Clinical Medicine, LKS Faculty of Medicine, HKU; Honorary Consultant (Surgery), Queen Mary Hospital.
Professor Feifei Wang, Assistant Professor, Department of Electrical and Electronic Engineering, Faculty of Engineering; and Materials Institute of Life Sciences and Energy (MILES), HKU.
Dr Ian Yu Hong Wong, Clinical Assistant Professor, Department of Surgery, School of Clinical Medicine, LKS Faculty of Medicine, HKU; Honorary Associate Consultant (Surgery), Queen Mary Hospital.
MILES PI


Hongjie Dai
Chair Professor, HKUNanoscience and nanomedicine; biomedical science and engineering, near-infrared-II/short wavelength infrared (NIR-II/SWIR) molecular imaging for cancer and immunology; nano-vaccines.



Feifei Wang
Assistant Professor, HKUAdvanced biomedical imaging, Near-infrared II fluorescence imaging, Super-resolution imaging.









